September 1, 2015 report
Functional catalyst for alternative fuel source by depositing nanosheets on a flexible carbon cloth
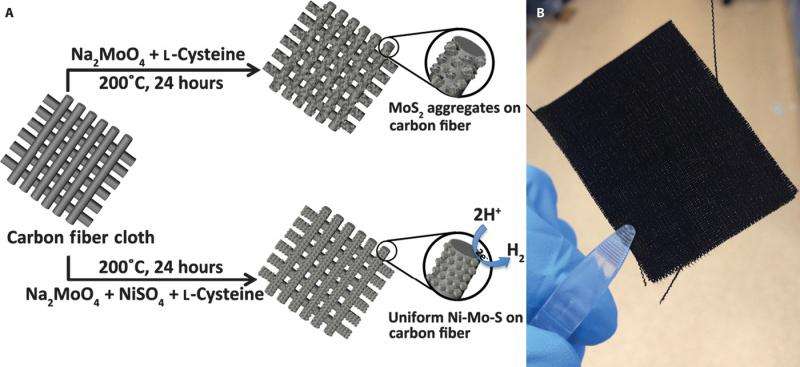
(Phys.org)—The past ten years have seen an uptick in research into hydrogen production through electrolysis as an alternative fuel source. In electrolysis water decomposes into its components, hydrogen gas and oxygen gas, environmentally friendlier gases compared to fossil fuel reactions. However, electrolysis of water requires additional energy, known as overpotenial, above beyond its thermodynamic reaction barrier. Catalysts, such as platinum, decrease the overpotential allowing the reaction to progress at a practical rate, but platinum is too expensive for use at an industrial scale prompting researchers to explore other types of electrocatalysts.
Researchers have explored molybdenum-based catalysts, particularly MoS2, as a possible alternative electrocatalyst for hydrogen evolution reactions. Studies of MoS2 nanoparticles demonstrate that catalysis occurs on edges or at defect sites. This means that controlling the MoS2 morphology could lead to controlling its catalytic activity. However, there are still several barriers to making MoS2 a feasible industrial material.
In new research reported in Science Advances, Jianwei Miao, Fang-Xing Xiao, Hong Bin Yang, Si Yun Khoo, Jiazang Chen, Zhanxi Fan, Ying-Ya Hsu, Hao Ming Chen, Hua Zhang, and Bin Liu from Nanyang Technological University in Singapore and National Taiwan University have devised a molybdenum-nickel-based hydrogen evolution catalyst that can be deposited on a flexible, conductive carbon fiber cloth. Analytical studies show that their Ni-Mo-S/C catalyst, when prepared using the reaction solution with a nickel-to-molybdenum ratio of 1:1, has better overpotential values and is more stable than MoS2 in neutral electrolyte.
Their synthesis for making the Ni-Mo-S/C catalyst is a straight-forward process that is biomolecule-assisted using L-cysteine as its sulfur source. They found that by adding Ni2+ to the initial solution, they were able to control the morphology of the nanoparticles deposited on the carbon cloth. They tested several different ratios of nickel-to-molybdenum, 3:1, 1:1, and 1:3, and confirmed via various methods that the Ni-Mo-S/C (1:1) was the best overall catalyst.
SEM and TEM showed that the Ni-Mo-S/C (1:1) provided the most consistent nanostructured film. Importantly this film resulted in more edges, as well as multiple pores, both of which should enhance catalytic activity. Other structures, including MoS2, formed unwanted aggregates when deposited on the carbon cloth. X-ray diffraction, energy dispersive x-ray spectroscopy, and XPS confirmed the chemical composition of their nickel-molybdenum catalyst and ensured that there were no nickel-sulfur bonds.
Miao, et al. conducted several voltammetry studies in both neutral and acidic electrolyte to test the catalytic behavior of their electrodes. Ni-Mo-S/C (1:1) showed the best hydrogen evolution catalytic activity with a smaller overpotential compared to MoS2/C and the other catalysts prepared with 3:1 and 1:3 nickel-to-molybdenum precursor ratio. Tafel plots showed that Ni-Mo-S/C (1:1) had the smallest slope which corresponds to a good reaction rate and low overpotential.
The authors wanted to qualitatively evaluate the potential at which catalysis occurs as well as gas evolution of different electrodes at the same time. They designed a special electrode comprised of single bundles of MoS2/C and Ni-Mo-S/C (1:1) attached to a home-made titanium connector. By linking these in parallel, they could apply a synchronized potential bias to both. Gas bubbles appeared first on the Ni-Mo-S/C (1:1) and were generated faster and more continuously along the bundle. MoS2 turned a pale color due to the adsorption of oxygen bubbles while the Ni-Mo-S/C (1:1) maintained the same physical appearance.
Finally, they tested catalysis stability using controlled potential electrolysis in a neutral electrolyte. Ni-Co-S/C (1:1) retains 97.5% of its initial current density after their initial test, while MoS2 maintains 85% of its current density. This is compared to a Pt electrode which has 15% of its current density after one hour.
Miao, et al.'s last experiment was to create a prototype of a hydrogen generator in the lab. The purpose of finding an alternative electrocatalyst is to identify one that that could feasibly be used in an industrial setting. They were able to role and shape their Ni-Mo-S/C (1:1) electrode to fit their apparatus and collected hydrogen gas in a relatively short amount of time at a potential bias of -0.5 V (vs. RHE). While this is a small prototype, it adequately demonstrates that this electrode could be a good candidate for larger scale hydrogen evolution reactions and is flexible enough to accommodate several hydrogen generator designs.
Overall, Ni-Mo-S/C (1:1) was made in a relatively straight-forward synthesis and all analyses point to its superior catalytic properties compared to MoS2 making it a robust alternative to a platinum catalyst.
More information: "Hierarchical Ni-Mo-S nanosheets on carbon fiber cloth: A flexible electrode for efficient hydrogen generation in neutral electrolyte" Science Advances, DOI: 10.1126/sciadv.1500259
Abstract
A unique functional electrode made of hierarchal Ni-Mo-S nanosheets with abundant exposed edges anchored on conductive and flexible carbon fiber cloth, referred to as Ni-Mo-S/C, has been developed through a facile biomolecule-assisted hydrothermal method. The incorporation of Ni atoms in Mo-S plays a crucial role in tuning its intrinsic catalytic property by creating substantial defect sites as well as modifying the morphology of Ni-Mo-S network at atomic scale, resulting in an impressive enhancement in the catalytic activity. The Ni-Mo-S/C electrode exhibits a large cathodic current and a low onset potential for hydrogen evolution reaction in neutral electrolyte (pH ~7), for example, current density of 10 mA/cm2 at a very small overpotential of 200 mV. Furthermore, the Ni-Mo-S/C electrode has excellent electrocatalytic stability over an extended period, much better than those of MoS2/C and Pt plate electrodes. Scanning and transmission electron microscopy, Raman spectroscopy, x-ray diffraction, x-ray photoelectron spectroscopy, and x-ray absorption spectroscopy were used to understand the formation process and electrocatalytic properties of Ni-Mo-S/C. The intuitive comparison test was designed to reveal the superior gas-evolving profile of Ni-Mo-S/C over that of MoS2/C, and a laboratory-scale hydrogen generator was further assembled to demonstrate its potential application in practical appliances.
Journal information: Science Advances
© 2015 Phys.org




















