Reaction snapshots of a notch-modifying enzyme provide a basis for drug design
Notch receptors are core components of the signaling pathways that regulate the development of cells within the human body. Notch signaling pathways can determine how cells proliferate or change during development, and defects in Notch signaling can lead to many diseases, including several types of cancer and developmental disorders.
New research by Huilin Li, a biochemist at the U.S. Department of Energy's Brookhaven National Laboratory and Stony Brook University, reveals intricate "stop-action" details of these reactions. Reporting on a series of structures of a Notch-modifying enzyme in different reaction states, Li's work captures the individual steps that glycosyltransferases – enzymes that catalyze sugar-based linkages – take, and how they retain a kind of handedness, or orientation, during the catalytic reaction. This work is published in the September 28 issue of Nature Chemical Biology.
"During development, Notch function is regulated by various sugar modifications." Li said. The key receptor in this pathway, Notch, is modified by different types of carbohydrates. Li studied how a specific enzyme adds a xylose – a type of sugar – to a repeat of the Notch receptor that is similar to epidermal growth factor, which stimulates cell growth. Understanding how these modifications work can identify new targets for drug development.
Visualizing the fleeting reaction process
The enzyme, called Xxylt1, adds xylose to Notch and can negatively regulate Notch signaling. Its gene is highly amplified in several cancers, including head and neck squamous cell carcinoma, lung squamous cell carcinoma, and esophageal carcinoma. When transferring a sugar, the enzyme retains its spatial arrangement, a kind of "handedness" or orientation, even after completing this process. That has left biochemists a bit mystified.
Two possible mechanisms are equally likely to preserve the handedness of the carbon molecule's orientation on the carbohydrate. One is the more classical double mirroring – a flip and then a flop, so that in the end, the molecule has the same spatial orientation it started with. The other is the rarely encountered SNi (Substitution Nucleophilic internal) mechanism – a sort of sneak attack from behind—where the reaction proceeds via a substitution in which the modified Notch receptor but not the enzyme launches the attack.
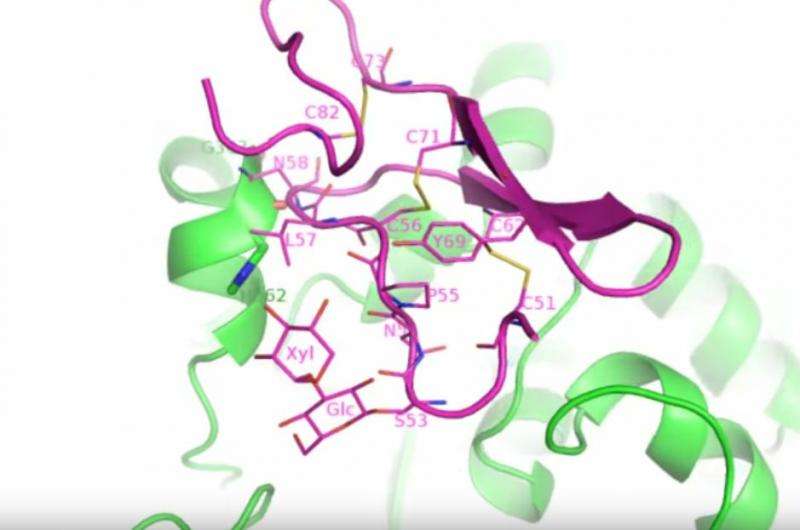
Determining which of the two mechanisms is actually used by retaining enzymes has been difficult because these reactions happen on such short timescales. But Li and his team were able to image the reaction as it happened because they stumbled upon a buffer – a solution used to hold the molecules stable – that slowed the reaction dramatically. As a result, they were able to use the intense X-rays at the National Synchrotron Light Source (NSLS) at Brookhaven National Laboratory to "take a series of snapshots that showed how the structure of the Notch receptor and this enzyme changed during the reaction," said Li. "We had a real stroke of luck."
Li collaborated with Robert Haltiwanger and Hideyuki Takeuchi, faculty members in Stony Brook University's Biochemistry Department at the time of the experiment, and currently at the University of Georgia. They were able to image several states during the reaction, including an unprecedented look at the state of the enzyme attached to the substrate it will modify just before the reaction starts. The snapshots were captured using NSLS beamlines X25, X29, and X6A.
"The fleeting reaction is usually difficult to trap through X-ray crystallography," Li said. "The reaction is slowed down in our experimental conditions. That's why we were able to take the series of snapshots to show the reaction as it progresses."
Li's team member, Hongjun Yu, solved these structures. "Our structures revealed the SNi-like retaining mechanism and disproved the double displacement mechanism," Yu said. "I was particularly excited to discover that the part of Notch that is recognized by and bound to our enzyme Xxylt1 could undergo such a dramatic structural change. This is unheard of and quite unexpected."
Their collaborators, Takeuchi and Haltiwanger, were equally excited by the finding. "When Hongjun showed us the distorted structure of EGF repeats bound to Xxylt1, Bob and I were stunned. People have thought that individual EGF repeats were very rigid and stiff, but it turned out that's not the case. The flexible nature of EGF repeats revealed by this study will provide totally novel insights into the mechanism of Notch receptor activation," Takeuchi said. "I am glad to see our collaboration with Li's group crystallize into this exciting paper."
Haltiwanger added, "The chemical mechanism for retaining enzymes such as Xxylt1 has generated intense debate in the field for several decades. The studies reported here resolve this conflict and provide essential information for the design of inhibitors that could potentially become therapies for Notch-related diseases."
More information: "Notch-modifying xylosyltransferase structures support an SNi-like retaining mechanism." Nature Chemical Biology (2015) DOI: 10.1038/nchembio.1927
Journal information: Nature Chemical Biology
Provided by Brookhaven National Laboratory