May 19, 2017 report
Catalyst for the carbon-free production of hydrogen gas from ammonia
(Phys.org)—Hydrogen has the potential to provide an alternative, clean energy source, particularly as applied to fuel cell technology. Current fuel sources involve carbon-containing fossil fuels or carbon-containing organic molecules, which result in the production of excess CO2, a greenhouse gas. Several initiatives, including a national initiative in Japan, seek to create a low-carbon usage society by using alternative fuel sources.
The Energy Carriers initiative in Japan is a national project that is specifically looking at ways to efficiently store and transport hydrogen. One way to do this is to use ammonia as a hydrogen source. However, discovery of an efficient process for breaking down ammonia has proved difficult, largely because the catalytic process to break down ammonia requires the continuous addition of heat, which can be prohibitively expensive.
Katsutoshi Nagaoka, Takaaki Eboshi, Yuma Takeishi, Ryo Tasaki, Kyoto Honda, Kazuya Imamura, and Katsutoshi Sato of Oita University in Japan have developed a method using a novel catalyst for producing hydrogen from ammonia without the addition of external heat through the catalytic cycle. Their work appears in Science Advances.
The decomposition of ammonia into hydrogen and nitrogen is an endothermic process, meaning that it requires the addition of energy to obtain products. This means that traditional catalytic decomposition reactions require the addition of a large amount of heat to obtain a useful amount of hydrogen gas.
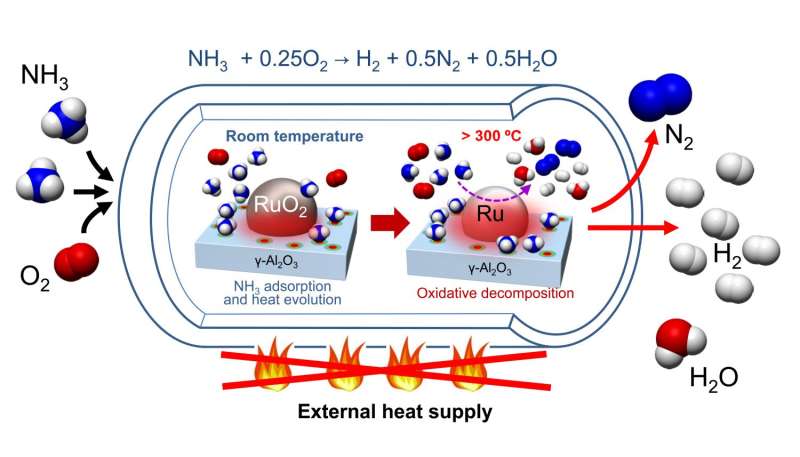
Nagaoka et al. developed a catalyst that is made of a RuO2 nanoparticle supported on γ-Al2O3 catalyst bed. After purging their catalyst of H2O and CO2, ammonia and oxygen were added to the reaction vessel where ammonia was adsorbed onto the catalytic surface, resulting in an increase in temperature. This increase in temperature catalyzed the oxidative decomposition of ammonia, an exothermic process. This heated up the reaction, which in turn, provided the energy for the endothermic decomposition of ammonia into hydrogen and nitrogen.
The catalyst pre-treatment did require heating to remove water and carbon dioxide, but it did not require subsequent re-heating. Tests on catalyst cycling showed that after the initial pre-treatment of the RuO2/γ-Al2O3 catalyst with helium at 300oC, the catalyst was able to cycle three times and still produce hydrogen in maximum yields. Furthermore, these studies included oxidative passivation to ensure that no heat was produced from oxidation of Ru to RuO2. In practice, oxidative passivation will not be necessary. So, even though heating is required to pre-treat the catalyst, heating is not required for additional cycles of the catalyst.
In an effort to understand how the RuO2/γ-Al2O3 catalyst works, Nagaoka et al. compared the maximum catalytic bed temperature that results from self-heating of RuO2/ γ-Al2O3 to RuO2/La2O3, a known ammonia decomposition catalyst. They found that the aluminum-based catalyst heated to a maximum temperature of 97oC, while the lanthanum-based catalyst heated to a maximum temperature of 53oC. This is important because the auto-ignition temperature for the oxidative combustion of ammonia is 90oC, and it explains why better reaction yields were seen with RuO2/ γ-Al2O3.
The authors point out that this difference in adsorption temperature is likely due to the favorable interaction between ammonia, a basic molecule, and Al2O3, which is a Lewis acid. La2O3, on the other hand, is a Lewis base.
Additionally, the authors looked at the difference between using bare γ-Al2O3 as a catalyst and RuO2/ γ-Al2O3. They found that 90% of the ammonia adsorbs onto bare γ-Al2O3 compared to the catalyst bed and the RuO2 nanoparticle. This implies that ammonia is chemisorbed onto the nanoparticle and γ-Al2O3, which then promotes multilayer physisorption.
Overall, this type of catalyst is helpful in providing enough heat to overcome the needed heat requirements for the endothermic decomposition of ammonia into hydrogen and nitrogen gas. This study shows that self-heating catalysis is a viable option for exploring solutions to the practical difficulties in using ammonia as a hydrogen fuel source.
More information: Katsutoshi Nagaoka et al. Carbon-free Hproduction from ammonia triggered at room temperature with an acidic RuO/γ-AlOcatalyst, Science Advances (2017). DOI: 10.1126/sciadv.1602747
Abstract
Ammonia has been suggested as a carbon-free hydrogen source, but a convenient method for producing hydrogen from ammonia with rapid initiation has not been developed. Ideally, this method would require no external energy input. We demonstrate hydrogen production by exposing ammonia and O2 at room temperature to an acidic RuO2/γ-Al2O3 catalyst. Because adsorption of ammonia onto the catalyst is exothermic, the catalyst bed is rapidly heated to the catalytic ammonia autoignition temperature, and subsequent oxidative decomposition of ammonia produces hydrogen. A differential calorimeter combined with a volumetric gas adsorption analyzer revealed a large quantity of heat evolved both with chemisorption of ammonia onto RuO2 and acidic sites on the γ-Al2O3 and with physisorption of multiple ammonia molecules.
Journal information: Science Advances
© 2017 Phys.org