Metal hydrides with improved stability in water
NUS chemists have discovered new reaction pathways using "acidic" metal hydrides to access industrially important chemical complexes.
Metal hydrides are compounds with the metal atoms bonded to hydrogen atoms, and are important for various catalytic and synthetic reactions. Mechanisms for the reaction pathways using traditional "basic" metal hydrides are well understood. However, their "basic" metal-hydrogen bond polarity causes them to react vigorously with water, often in an irreversible manner. This is undesirable for many industrial chemical reactions which are performed in aqueous solutions, or under "wet" atmospheric conditions, as a significant portion of the metal hydride catalyst will react with water molecules instead of the intended reactants. This constraint limits the use of metal hydrides in many industrial processes. Prof Rowan YOUNG and his research team from the Department of Chemistry, NUS have developed new "acidic" metal hydrides with an inverse metal-hydrogen bond polarity that allows them to be more stable in the presence of water. This important achievement has opened up avenues to new synthetic pathways that cannot readily be accessed via conventional metal hydrides.
For example, using the newly developed "acidic" metal hydrides, the research group discovered reaction pathways to important organometallic "pincer" ligand frameworks. These "pincer" complexes are of fundamental importance to modern catalysis and are an emerging class of metal complexes that have been very successful at promoting difficult bond activations and unique catalytic reactions, but are often difficult to access through traditional synthetic methods.
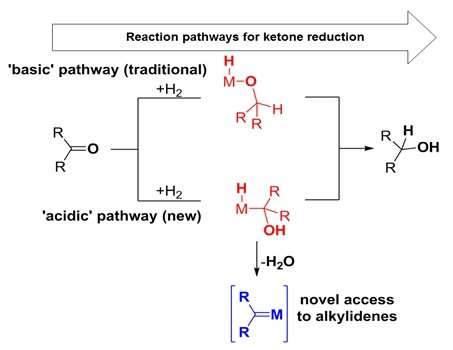
The Young group also exploited the "acidic" metal hydrides to map alternative reaction pathways (to previously understood mechanisms) for the hydrogenation of ketones. The hydrogenation of ketones is a key industrial reaction carried out by many chemical companies on a large scale throughout the world.

More information: Simon Sung et al. Convergent (De)Hydrogenative Pathways via a Rhodium α-Hydroxylalkyl Complex, Organometallics (2017). DOI: 10.1021/acs.organomet.7b00158
Simon Sung et al. Protonolysis of an α-Hydroxyl Ligand for the Generation of a PCcarbeneP Pincer Complex and Subsequent Reactivity Studies, Organometallics (2017). DOI: 10.1021/acs.organomet.7b00461
Simon Sung et al. Facile generation of iridium PCcarbeneP pincer complexes via water elimination from an alcohol proligand, Dalton Transactions (2017). DOI: 10.1039/C7DT03690F
Journal information: Dalton Transactions
Provided by National University of Singapore