Small changes to a surfactant's structure influence its ability to encapsulate oily molecules
The properties of surfactants, substances that lower the surface tension of a liquid, can be fine-tuned by tweaking their molecular structure, according to a recent A*STAR study. This method could help researchers develop better surfactants for a variety of applications, from aiding drug delivery, or improving the efficiency of oil drilling, to boosting the cleansing ability of soap.
Surfactants are molecules with split personalities. They typically have a hydrophilic 'head' that attracts water, and a hydrophobic 'tail' that prefers oily molecules. Surfactants can surround tiny oily droplets to form a structure called a micelle, which allows the oily molecules to be dispersed and stable in water.
Freda Lim and colleagues at the A*STAR Institute of High Performance Computing have now shown that rearranging the atoms in a common surfactant can have a big impact on its ability to form micelles.
The team performed computer simulations of a family of six different alkyl benzenesulfonate molecules, surfactants that, due to their cost-effectiveness and biodegradability, are widely used in the detergents and petroleum industries. These molecules sport alkyl 'tails' containing 12, 14 or 16 carbon atoms, and some have short alkyl groups in various positions on their benzenesulfonate 'heads'.
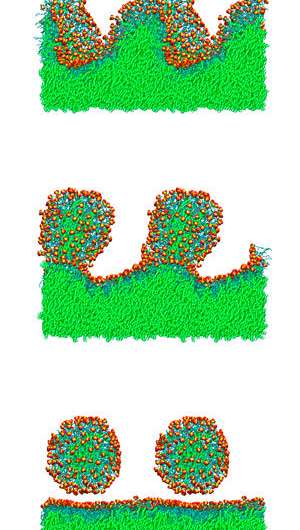
The researchers first simulated how the surfactants behaved in a single-molecule layer, trapped between water and a colorless, oily hydrocarbon called decane. As the concentration of surfactant molecules increased to the point where the layer was packed with surfactants, those with more compact heads and longer tails remained in a flat layer, while those with bulkier heads and shorter tails began to buckle into undulating waves. In general, the behavior of the surfactant also depended on the position of chemical groups around its head.
The researchers then continued to increase the concentrations of surfactants in the intermediate layer. Those with more compact heads and longer tails formed bud-like structures filled with decane, but did not release free micelles. In contrast, those with the bulkier heads and shorter tails formed buds that eventually broke free from the surfactant layer (see image).
"The selection of surfactants depends on the purpose for which it is used, so there is really no 'best' surfactant," explains Lim. "Our simulations provide a guideline on choosing the types of surfactants depending on the specific applications." The team now plans to study how different stimuli trigger the rupture of the surfactant micelle structures, and how the substances trapped within these structures can be released for applications.
More information: Jacqueline S. J. Tan et al. Interfacial Properties and Monolayer Collapse of Alkyl Benzenesulfonate Surfactant Monolayers at the Decane–Water Interface from Molecular Dynamics Simulations, Langmuir (2017). DOI: 10.1021/acs.langmuir.7b00171
Journal information: Langmuir