Restoring cells to an uninfected state once a virus is destroyed
New research, pioneered by a first year Ph.D. student and researchers at the University of St Andrews' School of Biology, has identified an important new component of the CRISPR genome engineering toolkit, which is revolutionising the treatment of genetic disease and infection.
The study, published in Nature (Wednesday 19 September 2018), focused on cell level defences against viral infections and the ability to restore cells to an uninfected state once a virus is destroyed. The research team, led by Professor Malcolm White from the Biomedical Sciences Research Complex (BSRC) at St Andrews, made the breakthrough, which could have wide range of applications in healthcare, biotechnology and agriculture.
All forms of life need to combat infection by viruses. Humans have an adaptive immune system based on antibodies that carries a memory of past infection and provides immunity. As with humans, microbes also have an adaptive immune system, the CRISPR system, which stores small pieces of viral genetic material that are used to provide immunity against future infection. The CRISPR system has been harnessed to manipulate and engineer genomes in the fight against infection and diseases.
Humans use the interferon pathway to signal infection and mobilise defences. Recently, an unexpected facet of the CRISPR system was discovered, whereby invading viral genetic material triggers the synthesis of a cyclic ring molecule. These rings, which consist of 4 or 6 linked adenosine monophosphate (AMP) molecules, are, like interferon, signalling molecules that push the cell into an antiviral state. They do this by activating a suite of degradative enzymes that destroy the invading viruses and provide immunity. However, if left in this activated state for too long, the cell could also die. To avoid this fate, cells were predicted to have a molecular "off switch" that removes the ring molecules once infection is cleared.
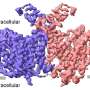
When he started his studies, first year Ph.D. student Januka Athukoralage set out to search for the elusive "off switch". He succeeded in purifying an enzyme, which he termed a "ring nuclease", that degrades the ring molecule specifically. Working with colleagues in the BSRC, he showed how the ring nuclease binds and chops up ring molecules, demonstrating how the "off switch" works to restore the cell to an uninfected state once the virus is destroyed.
Professor Malcolm White said: "The ring signalling mechanism is an important part of the CRISPR system that is used by microbes for antiviral defence. CRISPR has been harnessed as an exciting new technology to manipulate DNA, with applications in the treatment of genetic disease and infection, food production, rapid diagnostics and many other areas."
"The identification of the ring nuclease adds another piece to the CRISPR toolkit. This breakthrough was possible due to the hard work and expertise of a small team working together, and I'd like to acknowledge the crucial contributions of Drs Christophe Rouillon, Shirley Graham and Sabine Grüschow to this study."
Further studies are now underway to understand how the ring signalling system functions in cells.
More information: Januka S. Athukoralage et al. Ring nucleases deactivate type III CRISPR ribonucleases by degrading cyclic oligoadenylate, Nature (2018). DOI: 10.1038/s41586-018-0557-5
Journal information: Nature
Provided by University of St Andrews