Researchers report simpler method for safely handling methanethiol in chemical synthesis
The chemical element sulfur is an important constituent in many pharmaceuticals, and consequently, it is desirable to introduce sulfur-containing fragments efficiently in a broad range of chemical compounds. The Skrydstrup team has provided an effective way to introduce a small sulfur building block that is generally difficult to work with, a gas with an extremely repulsive odor. The team used a gold-based catalyst for reactions involving carbon-carbon double bonds.
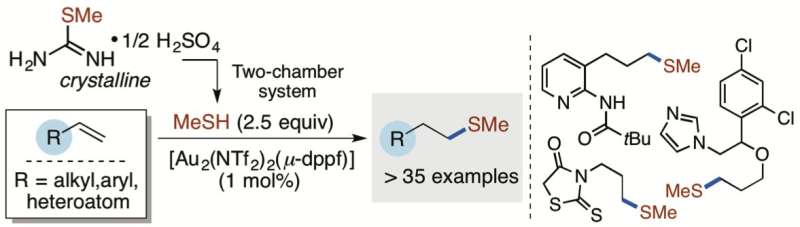
The research group focused on the use of the smallest carbon-containing thiol, methanethiol (MeSH). However, MeSH, the main compound responsible for bad breath and the smell of flatus, is also highly flammable and therefore unsafe to work with in the laboratory. In the present study, researchers from iNANO and the Department of Chemistry, Aarhus University, report that they successfully developed a two-chamber system in order to avoid handling pressure cylinders with MeSH or adding the gas directly to the chemical reactions. The authors also demonstrate that a crystalline organic compound can be used to liberate an exact amount of MeSH upon activation in the two-chamber system.
In this work, the direct use of MeSH was circumvented and a protocol for the delivery and use of a stoichiometric amount of gaseous MeSH has been developed without the need of pressure cylinders. The Skrydstrup group has demonstrated by the ex-situ generation of MeSH from a simple crystalline precursor in the two-chamber reactor that a gold(I)-mediated hydrothiolation of terminal alkenes can provide the corresponding methyl sulfide in high yields. The reaction promoted by a gold(I) complex is also interesting as these complexes appear to operate as radical initiators from the mechanistic investigation undertaken.
More information: Troels Skrydstrup et al, Ex Situ Formation of Methanethiol: Application in the Gold(I)-Promoted anti-Markovnikov Hydrothiolation of Olefins, Angewandte Chemie International Edition (2018). DOI: 10.1002/anie.201809051
Journal information: Angewandte Chemie International Edition
Provided by Aarhus University