From foe to friend: Graphene catalyzes the C-C bond formation
Graphene monolayers can be epitaxially grown on many single-crystal metal surfaces under ultra-high vacuum. On one side, these monolayers protect highly reactive metallic surfaces from contaminants, but on the other side, the piling of the layers as graphitic carbon blocks the activity of transition metal catalysts. The inertness of the graphite and the physical blockage of the active sites prevents chemical reactions occurring on the metal surface.
Researchers led by Fernando Martín, Emilio Pérez and Amadeo Vázquez de Parga (IMDEA Nanociencia and Universidad Autónoma de Madrid) have demonstrated that nanostructured graphene monolayers on a metal surface promote a chemical reaction that would be unlikely to take place under noncatalyzed conditions.
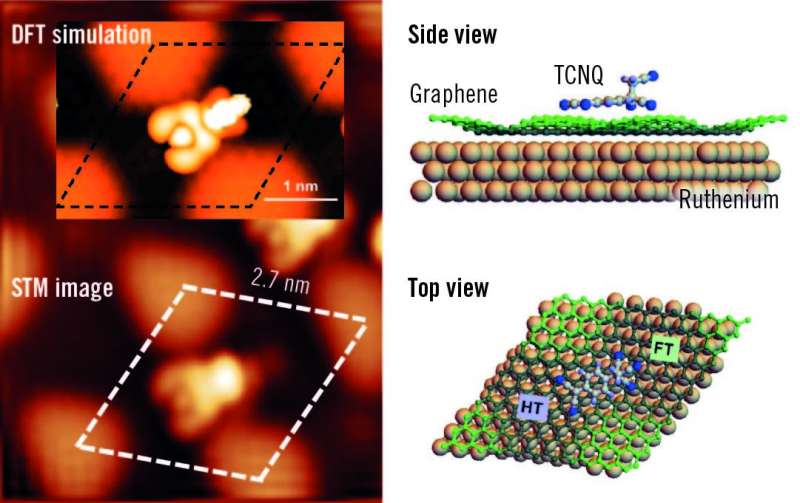
A crystal of ruthenium, Ru(0001), has been covered with an epitaxially grown continuous graphene layer. Because of the difference in lattice parameters, a new superperiodicity appears on the graphene layer and modulates its electronic properties. Taking advantage of the modulation, the surface has been functionalized with cyanomethylene groups (-CH2CN), covalently bonded to the center of the hexagonal close-packed areas in the Moiré unit cell, and doped with TCNQ (7,7,8,8-tetracyano-p-quinodimethane). TCNQ is an electron acceptor molecule used to p-dope graphene films.
When deposited on the graphene surface, this molecule is absorbed on a bridge position between two ripples. Here, it is worth noticing the important role of the surface and of the graphene layer in catalyzing the reaction of TCNQ and -CH2CN. The reaction of TCNQ with CH3CN (the pristine reactants are in gas phase) plus the loss of a hydrogen atom is very unlikely because of the high energy barrier (about 5 eV). The presence of the graphene layer reduces this energy barrier by a factor of 5, thus favoring the formation of the products.
The nanostructured graphene promotes the reaction in a threefold way: first, it holds the -CH2CN in place; second, it allows for an efficient charge transfer from the ruthenium; and third, it prevents the absorption of TCNQ by ruthenium allowing the molecule to diffuse on the surface. "
A similar clean reaction on pristine ruthenium is not possible, because the reactive character of ruthenium leads to the absorption of CH3CN and hinders the mobility of TCNQ molecules once absorbed on the surface" Amadeo says. The results confirm the catalytic character of graphene in this reaction. "Such a selectivity would be difficult to obtain by using other forms of carbon," Emilio confirms.
Further, the TCNQ molecules have been injected with electrons using the scanning tunneling microscope (STM). This individual manipulation of the molecules induces a C-C bond breaking, thus leading to the recovery of the initial reactants: CH2CN-graphene and TCNQ. The process is reversible and reproducible at a single-molecule level. As the researchers have observed a Kondo resonance, the reversibility of the process can be thought of as a reversible magnetic switch controlled by a chemical reaction.
More information: J. J. Navarro et al. Graphene catalyzes the reversible formation of a C–C bond between two molecules, Science Advances (2018). DOI: 10.1126/sciadv.aau9366
Journal information: Science Advances
Provided by IMDEA Networks Institute