One step closer to reality
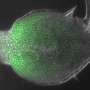
Diffusion is the process that lets the color spread through tea, but there is way more to it than that: It's also one of the most fundamental principles underlying the inner workings of living cells. The ability of molecules to move in or between cells determines where—and if—they can carry out their function. Consequently, the motility of molecules can reveal a lot about their tasks in the living organism. Scientists are therefore using so-called "FRAP" assays (FRAP: Fluorescence Recovery After Photobleaching) to investigate diffusion kinetics, a method established more than 40 years ago. The interdisciplinary team around Patrick Müller at the Friedrich Miescher Laboratory of the Max Planck Society in Tübingen, Germany, had a new take on this kind of experiment. In the journal Nature Communications they call attention to the limitations of pre-existing analysis tools for FRAP assays—and offer a flexible and accurate alternative: their open-access software "PyFRAP".
In FRAP assays, the time that fluorescent molecules need to replenish a bleached-out area is measured, basically assessing how quickly a dark sample area turns bright again. However, the evaluation of the resulting microscope images is anything but trivial: Molecular movement depends, among other things, on the shape of the environment. If a complex structure is approximated with oversimplified geometries to facilitate analysis, the estimated diffusion coefficients can be far off the actual values. PyFRAP operates without such simplistic assumptions and instead takes more realistic, three-dimensional structures into account. The program then numerically simulates the experiment and uses classical algorithms to fit the simulations to the measured data.
Dr. Alexander Bläßle, lead author of the publication, and his colleagues have identified a variety of potential problems with current FRAP analysis methods and addressed these during the development of PyFRAP. This thoroughness paid off: Compared to alternative programs, PyFRAP delivers particularly reliable results, especially under complicated conditions. And its flexible initial conditions also allow for evaluation of iFRAP data (iFRAP: inverse FRAP), a relatively new alternative to FRAP which is less harmful to delicate samples.
With the availability of a more precise analysis method, new applications for FRAP or iFRAP assays could now arise. The authors point out that their software can help to explore the interactions between molecules in living organisms: For example, it could help determine whether molecules are slowed down by interacting with (maybe so far undiscovered) binding partners.
PyFRAP has the potential to establish itself as a new standard analysis program in basic research. In any case, it already provides an impressive example of the benefits of constantly challenging established strategies and not being satisfied with simple, yet less accurate solutions.
More information: Alexander Bläßle et al, Quantitative diffusion measurements using the open-source software PyFRAP, Nature Communications (2018). DOI: 10.1038/s41467-018-03975-6
Software: The PyFRAP program is available to download free of charge at the following links:
www.fml.tuebingen.mpg.de/muell … -group/software.html
mueller-lab.github.io/PyFRAP/
Journal information: Nature Communications
Provided by Max Planck Society